 The FMH QuikQuant Kit is a flow cytometric method for FMH detection and quantification. The assay uses a reagent containing a monoclonal antibody to Hemoglobin F and Propidium Iodide as a specific marker of nucleated cells. The method is a rapid streamlined technique requiring about 30 minutes to complete. With less than 15 minutes of hands-on time needed, it is more efficient to use than the Kleihauer–Betke assay, as well as more sensitive and precise.
The FMH QuikQuant Kit is a flow cytometric method for FMH detection and quantification. The assay uses a reagent containing a monoclonal antibody to Hemoglobin F and Propidium Iodide as a specific marker of nucleated cells. The method is a rapid streamlined technique requiring about 30 minutes to complete. With less than 15 minutes of hands-on time needed, it is more efficient to use than the Kleihauer–Betke assay, as well as more sensitive and precise.
Key Benefits
- New technique is quicker to perform
- PI provides a definite tool for gating out the WBCs
- Kit includes 10X concentrated permeabilization reagent
- Works well with FETALtrol as a system control
Features
- Detection of fetal hemoglobin (HbF) of as low as 0,06% fetal cells in maternal blood
- Results in 1 hour (20 minutes hands-on-time) with two single centrifuge steps
- IVD/CE
Applications
- Determination of Fetomaternal Hemorrhage
- Pregnancy with suspected RhD incompatibilities
- Abdominal trauma
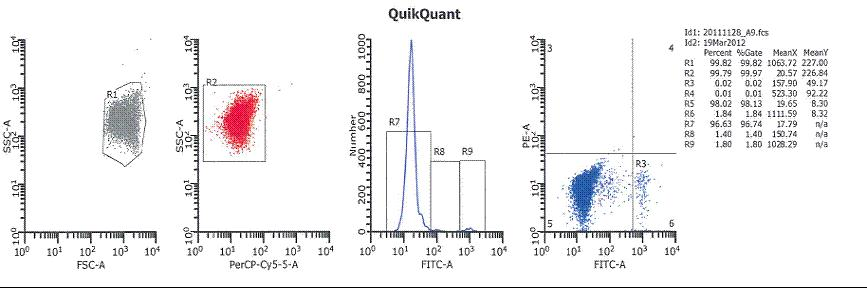 Figure 1: The figure above shows the results of the FMH QuikQuant . The red cells are the maternal RBCs, the blue cells are the maternal F- cells and the green cells are the HbF cells from the child.
Figure 1: The figure above shows the results of the FMH QuikQuant . The red cells are the maternal RBCs, the blue cells are the maternal F- cells and the green cells are the HbF cells from the child.
Product Information
FMH QuikQuant
Product Code: QQF-100
Size: 100 Test Kit
Regulatory Status: CE/IVD
Background Information
The most important clinical use of fetal RBC detection is the diagnosis and quantitation of fetomaternal hemorrhage (FMH). FMH occurs normally throughout pregnancy in minute amounts, with increasing volumes during the later stages of gestation [5]. If there is a significant difference in the RBC antigenicity between the fetus and mother, this can result in allosensitization of the maternal immune system either before or after parturition. The maternal antibodies to the fetal RBC antigens may be clinically silent or cause life-threatening autoimmune sequelae for the current or subsequent pregnancies (e.g.: erythroblastosis fetalis or early abortion). Such sensitization can occur with any RBC antigen mismatch, but the highest frequency and profound clinical consequences occur with Rh or D-antigen mismatches.
Detection and enumeration of Fetal RBCs is an essential part of the management of those patients with FMH treated with Rh immune globulin (RhIG) preparations. The use of Rh immune globulin prophylaxis is a universal practice, but dosing amounts and schedules have regional variations [7,8]. Hence, the sensitivity and specificity of detection assays for FMH is a critical factor in therapeutic efficacy and subsequent clinical outcome.
The most widely used assay for FMH detection has been the visual microscopic counting Kleihauer-Betke (KB) method, which is based upon the differences in solubility properties in acid conditions of fetal hemoglobin (HbF) from adult hemoglobin [9] While the KB method is easily performed by most clinical laboratories, it lacks sensitivity and exhibits poor reproducibility or precision (CVs of 50- 100%). Flow cytometric methods have been developed using the antigenic differences or quantitative assessment of fetal hemoglobin (HbF) to distinguish fetal RBCs from adult RBCs. These methods are more precise and less subjective. Nonetheless, many laboratories have continued to use the KB method due to the limited availability of Flow Cytometry.
For more information, references, and FAQs, please visit: http://www.iqproducts.nl/fmh-quikquant/
Also Available
FETALtrol – Tri-level stabilised blood controls containing known concentrations of human foetal erythrocytes in human adult blood.
The FMH Quikquant Kit & FETALtrol from IQ Products are distributed in the UK & ROI by Caltag Medsystems. If you would like further information about any of their products, please contact us at techsupport@caltagmedsystems.co.uk or by telephone 01280 827460.
